Multimodal Assessment of Ficus exasperata in Diabetic Drosophila
| Received 28 Mar, 2025 |
Accepted 24 Jun, 2025 |
Published 30 Jun, 2025 |
Background and Objective: The increasing occurrence of diabetes mellitus has spurred extensive research into natural products as potential alternative treatments. This research investigated the in vitro, in vivo, and in silico studies of the therapeutic potential of Ficus exasperata on diabetes. Aqueous and ethyl acetate extract fractions of Ficus exasperata were evaluated for antioxidant properties using standard protocols. Materials and Methods: Each of the fractions was administered at different concentrations to seven groups of Drosophila melanogaster . The in vivo inhibitory effects of the fractions were assayed for the expression of insulin-like peptide-2 (ILP-2), insulin-like receptor (InR), and Ecdysone inducible gene L2 (Imp-L2). The glucose concentration, non-protein thiol, total thiol, H2O2 , and nitrite activities were also evaluated. In silico studies assessed the inhibitory potentials of selected compounds on α-amylase as the target protein. All antioxidant studies were performed in triplicates, and data (Mean±SD) were statistically analyzed using one-way ANOVA with Duncan’s multiple range test (GraphPad Prism), considering p<0.05 as statistically significant. Results: The results revealed higher significant p<0.05 antioxidant contents in ethyl acetate fractions. Increased activities were also observed in rats treated with ethyl acetate fractions in comparison to acarbose administration. In silico analysis revealed naringenin and quercetin with docking scores of -7.118 and -5.898 kcal/mol, with binding energies of -41.806 and -31.715 kcal/mol, respectively, which competed with that of acarbose with the docking score and binding energy of -8.223 and -41.270 kcal/mol, respectively. Conclusion: However, the ADMET study also presented naringenin and quercetin with better physicochemical and pharmacokinetic properties. Gene expression profiling revealed ethyl acetate fractions with better upregulations in treated groups VI and VII in comparison to groups I and II. It could be recommended that ethyl acetate extract fractions of Ficus exasperata exhibited better potential efficacy in the treatment of diabetes and utility in enhancing the effectiveness of diabetic medications.
| Copyright © 2025 Shodehinde et al. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
INTRODUCTION
Diabetes mellitus (DM) is a metabolic condition characterized by elevated plasma glucose concentrations brought on by either insufficient or ineffective insulin. Numerous factors, including heredity and epigenetic propensity, lifestyle choices, and genetics, influence the release and function of insulin1. Diabetes was estimated to be the eighth most common cause of death and disability worldwide in 2019 by the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD), with over 460 million people of all ages and in all countries having the condition2. It is acknowledged that type 2 diabetes is a major public health issue that significantly affects both human life expectancy and medical expenses. In many regions of the world, the prevalence of diabetes is on the rise due to rapid urbanization and economic growth3. The prevalence and incidence of type 2 diabetes (T2DM) are escalating rapidly in Nigeria and across Sub-Saharan Africa, highlighting the urgent need for effective interventions4,5. Typical symptoms of T2DM include hyperglycemia, excessive urination (polyuria), increased thirst (polydipsia), and unintended weight loss. The disease primarily affects pancreatic β-cells, leading to dysregulated glucose levels, impaired absorption, and abnormal insulin production and function6.
Drosophila melanogaster, a fruit fly, is a useful model for examining the molecular causes of conditions like metabolic syndrome and type 2 diabetes7,8. These disorders share genetic and environmental factors that have been retained throughout evolution, and they are typified by metabolic abnormalities8. Insulin is essential for anabolism in mammals9 and has a major impact on fuel metabolism in Drosophila10. Important elements of the insulin signaling system in fruit flies include the ecdysone-inducible gene L2 (Imp-L2), insulin-like peptide-2 (Imp-L2), and the insulin-like receptor (InR). These molecules represent insulin receptors, Insulin-Like Growth Factor-Binding Proteins (IGFBPs), and vertebrate insulin, respectively11. In Drosophila, dietary changes can mimic human diabetes symptoms by causing obesity, metabolic dysfunction, and hyperglycemia12,13.
Diabetes is a complicated metabolic disease that can be effectively managed with the help of medicinal plants. Numerous traditional plants have been shown to have strong anti-diabetic effects without negative side effects. Flavonoids, alkaloids, phenolics, and tannins are among the many bioactive substances they contain. These compounds improve pancreatic function by either increasing insulin secretion or decreasing intestinal absorption of glucose14. It has been shown through experimental studies that about 410 medicinal plants have anti-diabetic compounds. However, the mechanisms of action of only 109 of these plants have been thoroughly investigated. The Krebs cycle, glycolysis, gluconeogenesis, glycogen synthesis and degradation, insulin synthesis and secretion, cholesterol synthesis, and carbohydrate metabolism and absorption are just a few of the metabolic pathways that have been demonstrated to be impacted by different plant extracts15. For the prevention and treatment of disease, plants and their bioactive compounds have been used extensively throughout history16.
Numerous illnesses in both people and animals have been successfully treated with medicinal plants. One such plant with a variety of therapeutic uses is the sandpaper leaf, or Ficus exasperata Vahl (FEVL)17. Ficus exasperata leaves exhibit a wide range of pharmacological effects, such as antiulcer, hypotensive, hypoglycemic, hypolipidemic, anti-inflammatory, anxiolytic, oxytocin-inhibiting, anticonvulsant, antinociceptive, antimicrobial, anticandidal, insecticidal, and pesticidal properties, according to reports from Western Nigeria18. Moreover, F. exasperata leaf decoctions and infusions have been used for many years in traditional medicine to treat and manage hypertension, diabetes mellitus, and other cardiovascular conditions19. This research explored the therapeutic potential of Ficus exasperata on diabetes through in vitro, in vivo, and in silico studies. Aqueous and ethyl acetate extract fractions of Ficus exasperata were assessed for their antioxidant properties using established standard protocols.
MATERIALS AND METHODS
Study area: The research was carried out in the Ondo State Region of Akungba-Akoko, a semi-rural settlement where the major occupation of the populace is farming and trading of food items. Ondo State has a boundary with neighboring states on the East-Edo and Delta, on the West-Ogun and Osun, on the North-Ekiti and Kogi, and South the bright Atlantic Ocean. Ondo State is located on the Latitudes 5°45' and 7°52' and Longitudes 4°20' and 6°05'E. Every analysis was carried out between the months of March-August, 2024, at the Adekunle Ajasin University, Department of Biochemistry in Akungba-Akoko, Ondo State, Nigeria.
Chemicals and reagents: All chemicals and reagents used for this study were of analytical grade, quercetin, gallic acid, ethanol, folin-ciocalteu reagent, Sodium Carbonate (Na2CO3), methanol, potassium acetate, Aluminum Chloride (AlCl3), potassium ferricyanide, trichloroacetic acid, DPPH (1,1-diphenyl-2-picrylhydrazyl), sodium chloride and Dinitrosalicyclic Acid (DNSA) reagent were purchased from Sigma-Aldrich, Inc. (St Louis, USA). Cornmeal, yeast, agar agar, sucrose, nipargin were purchased from a local market, and acarbose from a local pharmacy store. The distilled water used was obtained from the Biochemistry Department at Adekunle Ajasin University, Akungba Akoko, Ondo State, Nigeria. Optical absorbance was measured with a UV-visible spectrophotometer.
Plants collection: The leaf of Ficus exasperata was obtained from Akungba Akoko, Ondo State, Nigeria. The plant samples were subsequently identified and documented at the Plant Science and Biotechnology Departmental Herbarium (PSBH), Adekunle Ajasin University, Akungba-Akoko, Nigeria.
Fly stock and culture: The Drosophila melanogaster used in this study was obtained from the Drosophila Laboratory at the Department of Biochemistry, Faculty of Basic Medical Sciences, College of Medicine, University of Ibadan, Oyo State, Nigeria. The flies were maintained at a temperature of 25±2°C in the Drosophila Laboratory of the Department of Biochemistry, Adekunle Ajasin University, Akungba Akoko, Nigeria. The diet consisted of water, yeast, corn meal, brewer’s yeast, agar-agar, and Nipagin as a preservative, as well as the fly culture.
Composition of the feed: The composition of the formulated diet concerning the quantity of the ingredients is given below.
Preparation of the feed: The cornmeal was mixed thoroughly in 50 mL of water. Then 3 mL of ethanol was measured, and Nipargin was added to it, and the mixture was shaken. Then 100 mL of water was poured into the vessel to boil. After boiling the water, Agar was poured into the boiling water and mixed thoroughly using a stirring stick. Then, yeast was added and mixed immediately to avoid lumps. Then, the mixture of cornmeal was added to it and mixed thoroughly to avoid lumps. Then 50 mL of water was used to rinse the beaker, and poured it into the pot. Then, the mixture of nipargin and ethanol was added to it and left on fire for about 3 min. Then we poured it into the glass jar immediately before it thickened.
Acclimatization: The flies were fed for two weeks so that they could adapt to the new environment. The Drosophilas were fed with the feed as detailed in Table 1 and Fig. 1, but during the first 2 weeks, the Drosophilas were fed with the feed without the addition of extracts.
Methods
Preparation of extracts
Extraction and solvent-partitioned fractionation of crude extract: The leaves of Ficus exasperata were air-dried in a cool environment for 35 days and subsequently milled into powder using a mechanical grinder. A portion of the powdered sample (148 g) was extracted with ethanol (1000 mL) by soaking for 72 hrs. The ethanol extract was concentrated using a rotary evaporator, and the filtrate was allowed to dry over 2 weeks. The resulting extract (50 g) was dissolved in 500 mL of distilled water and partitioned with ethyl acetate in a separating funnel, yielding aqueous and ethyl acetate fractions. These fractions were dried and stored for further analysis.

|
| Table 1: | Composition of feed (250 mL of water) | |||
| Ingredient | Amount in diets |
| Corn meal | 8.3 g |
| Agar | 1.0 g |
| Yeast | 3.4 g |
| Nipargin | 1.0 g |
| Ethanol | 3 mL |
| Water | 200-250 mL |
In vitro antioxidant assays
Determination of total phenol content (TPC): Folin-Ciocalteu (FC) reagent (1:10v/v) of 2.5 mL was mixed with 0.5 mL of the fraction (1 mg/mL). After 5 min of incubation at 25°C, sodium carbonate in the amount of 2 mL (7.5%) was added. The mixture was measured at 765 nm after incubation at 40°C for 30 min. The standard used was gallic acid (0.02-0.1 mg/mL), and the fraction’s phenol was calculated as mg of gallic acid equivalent (mg GAE/g extract) using the standard curve20,21. The experiment was carried out in triplicate (n = 3).
Total flavonoid content: The total flavonoid content of each sample extract was determined according to the method of Meda et al.21. The appropriate volume (0.1 mL) of the fraction was mixed with 0.5 mL methanol, 50 μL of 10% Aluminum Chloride (AlC13), 50 μL of 1 M potassium acetate, and 1.4 mL of water. The reaction mixture was incubated at room temperature for 30 min. Thereafter, the absorbance of the reaction mixture was measured at 415 nm in the spectrophotometer.
Total terpenoid content: The total terpenoid content (TTC) of the extracts of Ficus exasperata was determined by the method of Ghorai et al.22. To 1 mL of the extracts, 2 mL of chloroform was added. The sample mixture was then vortexed thoroughly before being left for 3 min. Subsequently, 200 μL of concentrated Sulfuric Acid (H2SO4) was poured into the mixture, followed by incubation at room temperature for 1.5-2 hrs in the dark. A reddish-brown precipitate was formed in the mixture during incubation. After that, the supernatant was carefully decanted without disturbing the precipitation, and 3 mL of absolute methanol was added and vortexed well until complete dissolution of the precipitation in methanol. Absorbance was read at 538 nm using a visible spectrometer. The TTC of the extracts was calculated as mg of linalool per gram of extract (dry weight, DW). The equation of the standard curve was:
where, R2 = 0.9927.
Ferric reducing property (FRAP): The reducing property of the extracts will be determined according to the method described by Oyaizu23. A 2.5 mL aliquot was mixed with 2.5 mL of 200 mM sodium phosphate buffer (pH 6.6) and 2.5 mL of 1% potassium ferricyanide. The mixture was incubated at 50°C for 20 min. Then, 2.5 mL of 10% trichloroacetic acid was added. The mixture was centrifuged at 650 rpm for 10 min. As 5 mL of the supernatant was measured with an equal volume of water, and 1 mL of 0.1% ferric chloride. The absorbance was measured at 700 nm. The ferric-reducing antioxidant property was subsequently calculated.
DPPH free radical scavenging ability: The free radical-scavenging ability of each sample fraction against DPPH (1,1-diphenyl-2-picrylhydrazyl) free radical was evaluated according to the method of Gyamfi et al.24 with modifications. To 1 mL of 0.4 mM methanol solution of DPPH radicals, 0.1 mL of the sample extracts was added. The mixture was left in the dark for 30 min, and the absorbance was measured at 516 nm in the spectrophotometer.
In vitro assay of α-amylase inhibition
Determination of α-amylase inhibition: Appropriate dilutions of the extracts (500 μL) in 0.02M sodium phosphate buffer (pH 6.9 with 0.006M NaCl) were added to 0.5 mg/mL of homogenate pancreatic α-Amylase (EC 3.2.1.1) and subsequently incubated at 25°C for 10 min. Then, 500 μL of 1% starch solution in 0.02M sodium phosphate buffer (pH 6.9 with 0.006M NaCl) was added to each tube. The reaction mixture was incubated at 25°C for 10 min and stopped with 1.0 mL of dinitrosalicylic acid (DNSA) reagent. Therefore, the mixture was incubated in a boiling water bath for 5 min and cooled to room temperature. The reaction mixture was further diluted with distilled water, and the absorbance was read at 540 nm in a spectrophotometer. The percentage inhibition of the extracts was estimated using the following formula:
High-Performance Liquid Chromatography (HPLC) analysis: The ethyl acetate (EA) fraction was found to be the most active fraction based on the in vitro α-amylase inhibitory study. Therefore, the EA was subjected to HPLC analysis (Shimadzu Co., Kyoto, Japan) with a fluorescence detector (RF-10AXL), Prominence autosampler (SIL-20AHT; Shimadzu), Prominence pump (LC-20AD; Shimadzu), Hypersil GOLD column (4.6×250 cm, 5 mm; Thermo Fisher Scientific, Waltham, Massachusetts, USA), and column oven (CTO-10ASvp; Shimadzu). The syringe filter was used to filter the samples (fraction and reference compounds). Then, 50 mL of filtrate from each sample was inserted at a flow rate of 1 mL/min and running time of 50 min. To prepare the sample, 10 g of ethyl acetate fraction was dissolved in an amber bottle with 20 mL of acetonitrile/methanol. The mixture was vigorously shaken for 30 min. After shaking, the organic solvent was decanted into a 25 mL standard flask and filled up to the mark. Standard analytes were initially inserted into a chromatograph to generate a chromatogram with a peak profile used to create a window in the HPLC machine for analysis of the test sample. Then, an aliquot of the sample (EA) was inserted into the chromatograph to generate a corresponding peak profile of the sample in the chromatogram. The peak area and retention time of the sample were compared to those of the reference sample relative to the concentration obtained for the sample using the following formula:
Survival assay: Flies that are one to three days old were divided into 5 groups of 25 flies each. They were transferred under mild anesthesia using ice from culture vials into new diet vials for 45 days to avoid contamination and ensure good quality of diet throughout the experiment. The effects of the aqueous and ethyl acetate fractions on fly survival were investigated with a pilot study to determine the concentration of the fractions used. The experiment was done in triplicate:
| • | Group I: Basal diet (1 mL ethanol/10 g diet) | |
| • | Group II: 2 mg AQ/10 g diet | |
| • | Group III: 4 mg AQ/10 g diet | |
| • | Group IV: 2 mg EA/10 g diet | |
| • | Group V: 4 mg EA/10 g diet |
The EA and AQ concentrations were added to the fly diet every 5 days for 45 days, and fly mortality was documented daily for 45 days.
Induction of flies with diabetes using sucrose: The fruit flies were induced with type 2 diabetes according to the method of Omale et al.25 with slight modifications. The 2.5 g sucrose/10 g diet was added to the normal diet of the fly, with other diet components remaining constant (1% agar, 3.4% yeast, 8.3% corn meal, and 1% nipagin) to induce diabetes in the Harwich strain of D. melanogaster. The flies were exposed to sucrose incorporated in the diet for 10 days and observed for diabetes symptoms such as low rate of L3 larvae emergence, decreased body size, and decreased locomotive activities.
Experimental design: To assess the potential of Ficus exasperata in regulating glucose gene expressions in sucrose-induced diabetes in Drosophila melanogaster, the following experimental design was employed: Sucrose-induced flies were administered Ficus exasperata fractions, dissolved in aqueous (AQ) and ethyl acetate (EA) solvents, via their diet, with acarbose serving as the reference drug. The treatment spanned 10 days. The experiment was conducted in triplicate, with 25 flies per experimental group, with group 1, group 2, and group 3 functioning as normal control, negative control, and positive control, and arranged according to the design given below:
Control groups:
| • | Group I: Drosophila flies (DF) fed with basal diet and 1 mL of ethanol/10 g of diet | |
| • | Group II: Diabetic Drosophila flies (DF) with no treatment | |
| • | Group III: Diabetic Drosophila flies (DF) plus administered 25 mg acarbose/10 g diet |
Plant treated groups:
| • | Group IV: Diabetic Drosophila flies (DF) plus administered 2.0 g AQ/10 g diet | |
| • | Group V: Diabetic Drosophila flies (DF) plus administered 4.0 g AQ/10 g diet | |
| • | Group VI: Diabetic Drosophila flies (DF) plus administered 2.0 g EA/10 g diet | |
| • | Group VII: Diabetic Drosophila flies (DF) plus administered 4.0 g EA/10 g diet |
After the treatment of flies was carried out for 10 days, the flies were anesthetized using ethanol. The weight of flies was taken at a ratio of 1 mg of flies/10 mL of buffer were homogenized using 0.1 M phosphate buffer (pH 7.0). The homogenates were centrifuged for 10 min at 4000x g, and the supernatants were separated from the pellets. The supernatants were kept and used for glucose and in vivo antioxidant assays.
Locomotor assay: The locomotor assay was employed to measure negative geotaxis according to the method of Adedara et al.26. Ten normal flies and experimental flies were briefly anesthetized under ice and transferred to a 15×15 cm glass column. After recovery, the flies were gently tapped down the column. The number of flies that were able to climb up to the 6 cm mark was recorded. The percentage of negative geotaxis was estimated using the following formula:
Estimation of glucose concentration: The assay for determining the concentration of glucose in the fly homogenate was conducted according to the procedure of Trinder27 using the Agappe LiquiCHEK Kit.
Estimation of total thiol content: The total thiol level was assayed according to the method of Ellman, as described by Abolaji et al.28 with some modifications. The total reaction mixture of 600 μL containing 25 μL of the sample, 510 μL potassium phosphate buffer (pH 7.4), 30 μL DTNB, and 30 μL GSH was incubated for 30 min at 25°C, and the absorbance was read at 412 nm using a spectrophotometer. A GSH standard curve was prepared to extrapolate the total thiol content, and the results were calculated per mg protein content.
Estimation of non-protein thiol content: About 4% Sulpho-salicyclic acid and the sample of the same ratio (1:1) were added together and allowed to incubate for 1 hr, and were centrifuged at 5000 rpm for 10 min at 4°C. About 1700 μL of 0.1 M potassium phosphate was added to 200 μL of supernatant and 100 μL of 10 mM DTNB. Then it was incubated for 30 min, then the absorbance was taken at 412 nm. Reduced glutathione (GSSG) was used as a standard.
Estimation of nitric oxide level: The nitric oxide (NO) concentration was estimated by the Griess reaction procedure as reported by Green et al.29. The mixture of homogenate and Griess solution was allowed to stand for 20 min at 27°C. Thereafter, the absorbance was read at 550 nm.
The level of NO in the mixture was extrapolated from the NaNO2 calibration curve.
Estimation of hydrogen peroxide level: The Hydrogen peroxide concentration was estimated according to the method described by Patterson et al.30. Ficus exasperata extract (0.5 g) was mixed with 5 mL of 5% TCA and 0.15 g activated charcoal. The mixture was centrifuged at 10,000 g for 20 min at 4°C. The supernatant was adjusted to pH 8.4 with 17 M ammonia solution and then filtered. The filtrate was divided into aliquots of 1 mL. To one of these, the blank was added 8 μg of catalase and then kept at room temperature for 10 min. To both aliquots with and without catalase, 1 mL of colorimetric reagent was added. The reaction solution was incubated for 10 min at 30°C. Absorbance at 505 nm was determined spectrophotometrically. The colorimetric reagent contained 10 mg of 4-aminoantipyrine, 10 mg of phenol, 5 mg of peroxidase (150 U/mg), dissolved in 50 mL of 100 mM acetic acid buffer (pH 5.6).
Gene expression study
Isolation of total RNA: Total RNA was isolated from whole Drosophila samples with the Quick-RNA MiniPrep™ Kit (Zymo Research). The DNA contaminant was removed following DNase I (NEB, Cat: M0303S) treatment. The RNA was quantified at 260 nm, and the purity was confirmed at 260 and 280 nm using an A&E Spectrophotometer (A&E Lab. UK).
cDNA conversion: One (1 μg) of DNA-free RNA was converted to cDNA by reverse transcriptase reaction with the aid of a cDNA synthesis kit based on ProtoScript II first-strand technology (New England BioLabs) in a condition of 3-step reaction: 65°C for 5 min, 42°C for 1 hr, and 80°C for 5 min.
PCR amplification and agarose gel electrophoresis: Polymerase Chain Reaction (PCR) for the amplification of gene of interest was carried out with OneTaqR2X Master Mix (NEB) using the following primers (Inqaba Biotec, Hatfield, South: Africa):set: >NM_079288.3 Drosophila melanogaster Insulin-like peptide 2 (Ilp2), mRNA Insulin-like peptide-2 Forward primer: AGGTGCTGAGTATGGTGTGC Reverse primer: TGTCGGCACCGGGCAT, >NM_001274480.1 Drosophila melanogaster Ecdysone-inducible gene L2 (ImpL2), transcript variant D, mRNA Forward primer TGCCGGCTTAGACACTAATTT Reverse primer GCTGCCTGAATC GCTAGGAA, >NM_001144622.2 Drosophila melanogaster Insulin-Like Receptor (InR), transcript variant C, mRNA Forward primer TTTGTGCCTCGCACTTTGC Reverse primer ATACGCTCACCAACACATGC, >NM_001273184.2 Drosophila melanogaster Glycerol-3-Phosphate Dehydrogenase (Gpdh), transcript variant G, mRNA, mRNA Forward primer TCGGACTGCGTAGACACTAGA Reverse primer AGCGCCATCTATGT AAGGATGT. The PCR amplification was performed in a total of 25 μL reaction mixture containing cDNA, primers (forward and reverse), and Ready Mix Taq PCR master mix. Under the following conditions: Initial denaturation at 95°C for 5 min, followed by 30 cycles of amplification (denaturation at 95°C for 30 sec, annealing for 30 sec, and extension at 72°C for 60 sec) and ending with final extension at 72°C for 10 min. The amplicons were resolved on a 1.0% agarose gel. The GAPDH gene was used to normalize the relative level of expression of each gene, and quantification of band intensity was done using ImageJ software.
In silico ADME toxicity screening and drug-likeness prediction: The ADMET SAR 1.0 and SwissADME platforms were used to predict the pharmacokinetic and pharmacodynamic properties of the compounds, including toxicity profiles and drug-likeness.
Ficus exasperata compounds library, target proteins, and molecular docking procedures: The 3D structures of compounds characterized from Ficus exasperata through HPLC analysis were retrieved from the PubChem database. These ligands were subsequently optimized and refined. The target protein, alpha-amylase (PDB ID: 1CLV), was obtained from the Protein Data Bank and prepared for molecular docking following the procedure described by Balogun et al.31. A total of nineteen bioactive compounds from F. exasperata were docked into the active site of the target protein, defined by its co-crystallized ligands. The refined ligands and protein structures were subjected to molecular docking to determine the biochemical interactions and potential mechanisms of action. Protein-ligand interactions and binding conformations were visualized using the Ligand Interaction Module of Schrodinger.
Statistical analysis: Triplicates of all antioxidant studies were carried out. The data was statistically analyzed using GraphPad Prism. The data was presented as a Mean Standard Deviation (SD). The statistical analyses employed One-way Analysis of Variance (ANOVA) with multiple comparisons, and Duncan’s multiple range tests to compare the means. The p<0.05 was used to evaluate statistical significance.
RESULTS AND DISCUSSION
The polyphenolic content and antioxidant activity of Ficus exasperata leaf extracts varied significantly between the aqueous and ethyl acetate solvents. The ethyl acetate extract exhibited the highest levels of total phenols (196.10±4.19 mg/g), flavonoids (2.63±0.07 mg/g), and terpenoids (96.85±1.98 mg/g), compared to the aqueous extract, which showed 55.05±5.29, 1.24±0.10, and 32.96±1.00 mg/g, respectively. Similarly, antioxidant activity assessed by FRAP and DPPH assays revealed stronger activity in the ethyl acetate extract, with a FRAP value of 1.15±0.01 mM Fe2 and 83.41±0.35% DPPH inhibition, in contrast to 0.90±0.01 mM Fe2 and 68.69±2.42% inhibition in the aqueous extract. These findings indicate that ethyl acetate is a more effective solvent for extracting polyphenolic compounds and enhancing antioxidant activity in F. exasperata leaves shown in Table 2. Drosophila melanogaster. However, the ethyl acetate extract had higher inhibitory activity aqueous extract.
| Table 2: | Polyphenolic content and antioxidant activity of F. exasperata leaf | |||
| Parameter | Total phenol (mg/g) | Total flavonoid (mg/g) | Total terpenoid (mg/g) | FRAP (mM Fe2+) | DPPH (%) |
| Aqueous (mg/g) | 55.05±5.29 | 1.24±0.10 | 32.96±1.00 | 0.90±0.01 | 68.69±2.42 |
| Ethyl acetate (mg/g) | 196.10±4.19 | 2.63±0.07 | 96.85±1.98 | 1.15±0.01 | 83.41±0.35 |
| Result shows Mean±SE, where, n = 3, FRAP: Ferric reducing antioxidant property and DPPH: 2,2-diphenyl-1-picrylhydrazyl | |||||
| Table 3: | Phenolics and flavonoids composition of Ficus exasperata leaf | |||
| Parameter | Percentage |
| Alpha-caryophyllene | 1.266±0.03 |
| Isoquinoline | 2.750±0.02 |
| Garcinol | 4.450±0.02 |
| Caffeic acid | 5.466±0.01 |
| Catechin | 6.483±0.02 |
| Epigallocatechin | 7.333±0.02 |
| Stigmasterol | 7.950±0.01 |
| Sitosterol | 8.816±0.01 |
| Orientin | 9.350±0.02 |
| Quercetin | 11.050±0.03 |
| Kaempferol | 12.166±0.01 |
| Rutin | 13.700±0.02 |
| Lupeol | 16.250±0.02 |
| Linalool | 17.616±0.03 |
| Naringenin | 18.900±0.02 |
| Hesperidin | 19.250±0.02 |
| Isovitexin | 19.683±0.02 |
| Vitexin | 20.500±0.02 |
| Isorhamnetin | 21.133±0.02 |
| Values represent Means±Standard Deviations of triplicate readings | |
| Table 4: | Binding energy scores and MMGBSA between targets and Ficus exasperata leaf compounds | |||
| Compound | Docking score (kcal/mol) | MMGBSA |
| Naringenin | -7.118 | -41.27 |
| Quercetin | -5.898 | -31.715 |
| Catechin | -4.953 | -25.324 |
| Kaempferol | -4.814 | -27.561 |
| Acarbose | -9.337 | -42.27 |
The phytochemical profiling of Ficus exasperata leaf revealed a diverse composition of phenolics and flavonoids, with isorhamnetin (21.133±0.02%), vitexin (20.500±0.02%), and isovitexin (19.683±0.02%) present in the highest concentrations, followed by hesperidin (19.250±0.02%) and naringenin (18.900±0.02%). Other notable compounds included linalool (17.616±0.03%), lupeol (16.250±0.02%), and rutin (13.700±0.02%). Flavonoids such as kaempferol (12.166±0.01%), quercetin (11.050±0.03%), and orientin (9.350±0.02%) were also abundant, indicating the plant’s rich antioxidant potential shown in Table 3.
In silico molecular docking results demonstrated that naringenin had a favorable binding affinity with a docking score of -7.118 kcal/mol and a strong binding free energy (MMGBSA) of -41.270, close to that of the standard drug acarbose (-9.337 kcal/mol, -42.270 MMGBSA). Quercetin, catechin, and kaempferol also showed notable binding scores of -5.898, -4.953, and -4.814 kcal/mol, respectively. These findings suggest that certain flavonoids in F. exasperata may have promising therapeutic interactions with target biomolecules, supporting their pharmacological relevance shown in Table 4.
The result depicts the information about the lipophilicity (Log P) and water solubility (Log Sw) of five compounds. The result indicated that all the compounds have a balanced character, with good solubility in water. The drug-likeness and bioavailability predictions for the same five compounds suggested characteristics that are typically associated with successful oral absorption and bioavailability. The physicochemical analysis of selected Ficus exasperata leaf compounds revealed that all tested phytochemicals-including naringenin (418.39 g/mol), quercetin (290.27 g/mol), catechin (302.24 g/mol), and kaempferol (286.24 g/mol)-fall within a suitable molecular weight range and are classified as soluble based on Silicos-IT predictions. Their consensus Log P values ranged from 0.73 (naringenin) to 1.58 (kaempferol), indicating moderate lipophilicity, while Log Sw values varied from -2.14 to -3.82, supporting their solubility. Although acarbose had a higher molecular weight (645.60 g/mol) and a highly negative Log P (-6.22), it also remained soluble, with a positive Log Sw value (6.40), underscoring its hydrophilic nature. These profiles suggest favorable drug-like properties for the phytochemicals compared to the reference compound, acarbose, in Table 5.
| Table 5: | Lipophilicity (Log P) and water solubility (Log Sw) prediction with SwissADME | |||
| Compound | Molecular weight | Consensus Log P | Silicos-IT logSw | Silicos-IT class |
| Naringenin | 418.39 | 0.73 | -2.28 | Soluble |
| Quercetin | 290.27 | 0.85 | -2.14 | Soluble |
| Catechin | 302.24 | 1.23 | -3.24 | Soluble |
| Kaempferol | 286.24 | 1.58 | -3.82 | Soluble |
| Acarbose | 645.6 | -6.22 | 6.4 | Soluble |
| Table 6: | Drug-likeness and bioavailability prediction, acarbose, however, violates three of the Lipinski rules, indicating potential challenges with its bioavailability according to these criteria | |||
| Compound | Lipinski #violations | Bioavailability score |
| Naringenin | 0 | 0.55 |
| Quercetin | 0 | 0.55 |
| Catechin | 0 | 0.55 |
| Kaempferol | 0 | 0.55 |
| Acarbose | 3 | 0.17 |
The drug-likeness and oral bioavailability of the selected Ficus exasperata compounds were evaluated based on Lipinski’s Rule of Five and bioavailability scores. Naringenin, quercetin, catechin, and kaempferol showed no violations of Lipinski’s rules, each with a bioavailability score of 0.55, indicating good oral absorption potential. In contrast, acarbose violated three of Lipinski’s criteria and exhibited a lower bioavailability score of 0.17, suggesting potential limitations in oral bioavailability despite its pharmacological effectiveness. These results support the suitability of the plant-derived compounds as promising drug-like candidates with favorable pharmacokinetic properties in Table 6.
The ADMET analysis revealed varying gastrointestinal (GI) absorption and enzyme inhibition profiles among the tested compounds. Quercetin, catechin, and kaempferol demonstrated high GI absorption, whereas naringenin and acarbose showed low absorption. None of the compounds were predicted to permeate the blood-brain barrier (BBB). Naringenin, quercetin, and acarbose were identified as P-glycoprotein (Pgp) substrates, while catechin and kaempferol were not. Notably, catechin and kaempferol were predicted to inhibit multiple cytochrome P450 enzymes (CYP1A2, CYP2D6, and CYP3A4), suggesting potential drug-drug interaction concerns. In contrast, naringenin, quercetin, and acarbose showed minimal or no CYP inhibition.
Toxicity predictions using ProTox II indicated that quercetin and acarbose belong to the safest toxicity class (Class 6), with high LD50 values of 10,000 and 24,000 mg/kg, respectively. Naringenin (LD50: 2300 mg/kg) and kaempferol (LD50: 3919 mg/kg) were classified under Class 5, while catechin showed higher toxicity (Class 3) with an LD50 of 159 mg/kg. Hepatotoxicity was only predicted for acarbose, and immunotoxicity was observed for naringenin and acarbose. Catechin displayed potential carcinogenicity and mutagenicity. None of the compounds showed cytotoxicity except catechin, which exhibited moderate toxic effects.
These results suggest that while plant-derived compounds such as quercetin, kaempferol, and naringenin have favorable ADMET and safety profiles, catechin and acarbose pose higher risks due to toxicity or metabolic interactions in Table 7 and 8.
| Table 7: | ADMET property prediction | |||
| Compound | GI absorption |
BBB permeant |
Pgp substrate |
CYP1A2 inhibitor |
CYP2C19 inhibitor |
CYP2C9 inhibitor |
CYP2D6 inhibitor |
CYP3A4 inhibitor |
| Naringenin | Low | No | Yes | No | No | No | No | No |
| Quercetin | High | No | Yes | No | No | No | No | No |
| Catechin | High | No | No | Yes | No | No | Yes | Yes |
| Kaempferol | High | No | No | Yes | No | No | Yes | Yes |
| Acarbose | Low | No | Yes | No | No | No | No | No |
| Table 8: | Toxicity prediction by ProTox II | |||
| Compound | LD50 (mg/kg) | Toxicity class | Hepatotoxicity | Carcinogenicity | Immunotoxicity | Mutagenicity | Cytotoxicity |
| Naringenin | 2300 | 5 | - | - | + | - | - |
| Quercetin | 10000 | 6 | - | - | - | - | - |
| Catechin | 159 | 3 | - | + | - | + | - |
| Kaempferol | 3919 | 5 | - | - | - | - | - |
| Acarbose | 24000 | 6 | + | - | + | - | - |
| +: Compound is predicted to have that type of toxicity and -: Compound is predicted not to have that type of toxicity | |||||||
The study investigated the effect of Ficus exasperata leaf extracts on glucose and thiol levels in diabetic D. melanogaster. Diabetic D. melanogaster flies were used as a model organism to test the potential of Ficus exasperata extracts as a natural treatment for diabetes. The antioxidant properties of two different leaf fractions, aqueous and ethyl acetate fractions of F. exasperata, were evaluated and several parameters to assess the antioxidant activity of each fraction were measured, which include total phenol content, total terpenoid, total flavonoid content, ferric reducing antioxidant property, and DPPH radical scavenging ability.
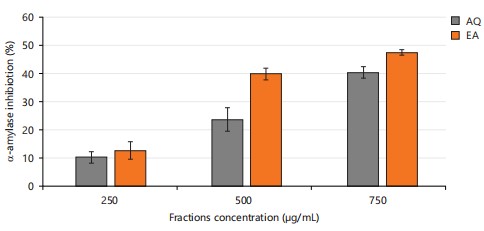
Total phenol content is a measure of the number of phenolic compounds in a sample31. Phenolic compounds are known to have antioxidant properties, so a higher total phenol content is generally considered to be an indicator of stronger antioxidant activity, which agrees with the reported work of Noreen et al.32. Flavonoids are recognized for their diverse biological activities, including antioxidant, anti-inflammatory, and anticancer properties, and play an essential role in safeguarding cells from oxidative damage33. In Table 2, all ethyl acetate fractions had significantly higher (p<0.05) phenolic content and antioxidant properties compared to the aqueous fractions. As shown in Fig. 2, the ethyl acetate fraction showed a stronger inhibitory activity at different concentrations against alpha amylase, one of the key enzymes involved in carbohydrate digestion. This suggests that the ethyl acetate fractions have stronger antioxidant activity, potential to inhibit carbohydrate absorption than the aqueous fractions, indicating that F. exasperata possesses medicinal properties with a promising therapeutic value.
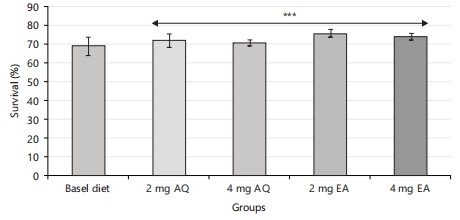
The survival rates of the different groups were recorded and analyzed using statistical methods. The statistical analysis (Fig. 3) revealed no significant difference in the survival rates of the different groups (p<0.05). This suggests that the aqueous and ethyl acetate fractions of F. exasperata had a significant effect on the survival of the flies. This agreed with the reported work of Balogun et al.31 in which Drosophila flies were subjected to experimental conditions that test for survival rates using Ficus exasperata before proceeding with further studies.
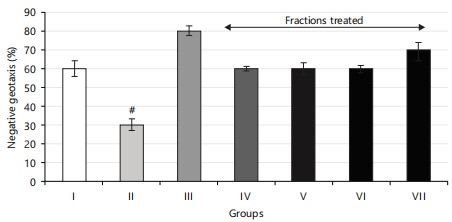
The negative geotaxis results observed in the flies validated the concentrations of fractions utilized in this study (Fig. 4). A notable (p<0.05) increase in climbing (locomotor) activity was recorded in both normal flies and diabetic flies treated with diets containing aqueous and ethyl acetate extract fractions compared to untreated diabetic flies. The restorative effects observed with aqueous and ethyl acetate extract fractions were linked to their antioxidant properties.
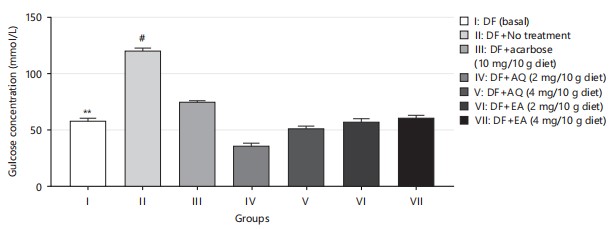
One of the therapeutic strategies for managing type 2 diabetes involves controlling glucose absorption by reducing starch hydrolysis through the inhibition of pancreatic α-amylase, thereby limiting glucose uptake33,34. The antihyperglycemic effects of diets enriched with aqueous and ethyl acetate extract fractions of F. exasperata suggest their potential antidiabetic properties, which may be attributed to the presence of bioactive phytochemicals in the plant. However, in Fig. 5, there was a significant difference between the normal control and non-treatment group, and the comparison between the non-treatment group and treatment group also shows a significant difference. There was a reduction in glucose concentration in the group treated with aqueous fraction when compared to its ethyl acetate fraction counterpart, reinforcing its potential to reduce glucose levels and indicating promising antidiabetic effects.
|
|
|
|
|
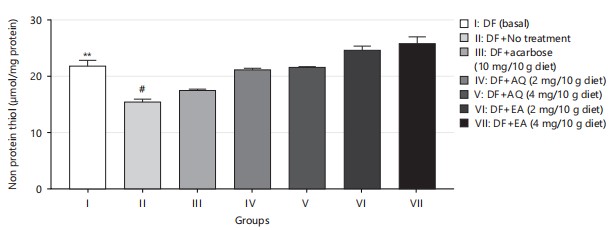
Non-protein thiols play an important role in protecting cells from oxidative stress35, which is a major contributor to diabetes and its complications. The amelioration of non-protein thiol levels, as shown in Fig. 6, suggests that both plant extracts have a protective effect against oxidative stress in diabetic flies. This protective effect could potentially translate to humans with diabetes as well, indicating the potential of F. exasperata as a natural therapy for diabetes and its complications.
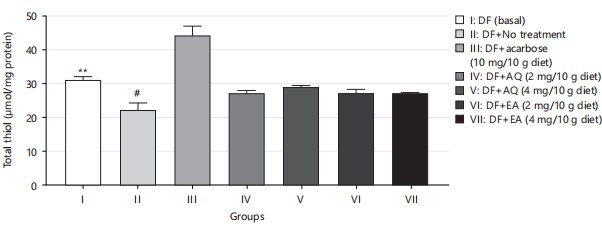
The total thiol concentration is an important parameter for assessing the antioxidant status of a biological system. Thiols are important components of the antioxidant defense system as they can directly scavenge free radicals or regenerate other antioxidants such as glutathione36. The decrease in total thiol concentration in diabetic flies (group II) compared to the control (group I) suggests that diabetes is associated with a decrease in the antioxidant defense system, which may contribute to the development of diabetic complications (Fig. 7). The augmented total thiol concentration in the diabetic flies treated with aqueous and ethyl acetate fractions (groups IV-VI) compared to the untreated diabetic flies (group II) and compared with the drug-treated flies in group III suggests that these fractions have antioxidant activity and may help to restore the antioxidant defense system. The decrease in total thiol concentration of diabetic flies is in agreement with the results of Prakash et al.36.
|
|
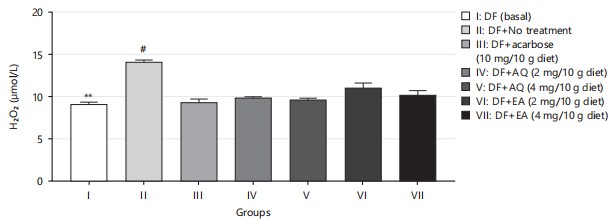
In this study, the reduction in the generation of H2O2 observed in diabetic flies on exposure to various fractions and doses of the plant extracts (Fig. 8) corroborates the earlier findings that cells can be maintained when free radicals such as H2O2 are kept under control37.
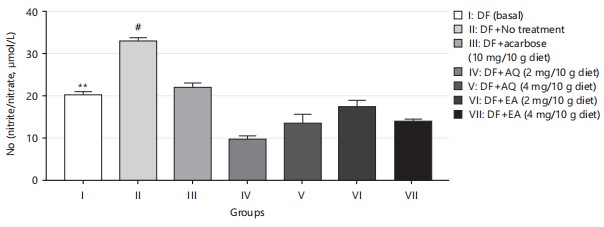
Nitric oxide (NO) is an essential regulatory molecule with significant cellular and metabolic functions. Managing NO metabolism is crucial in type 2 diabetes, as insulin plays a role in activating NO synthase38. An increase in NO concentration, along with hydrogen peroxide production, as shown in Fig. 8 and 9, indicates oxidative damage. The significant reduction of NO levels in HDS-induced diabetic flies by AQ and EA further supports their antioxidant potential.
|
|
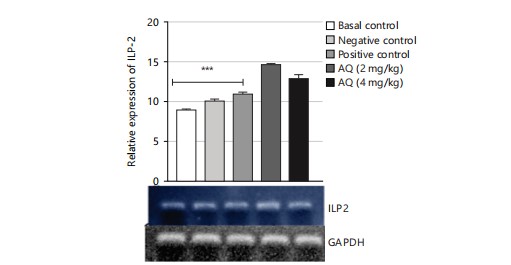
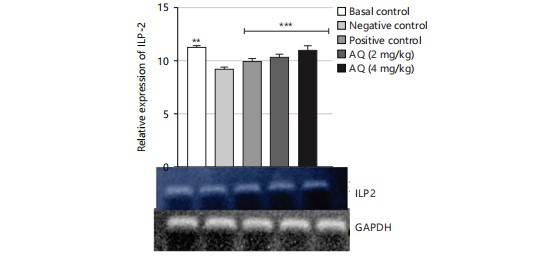
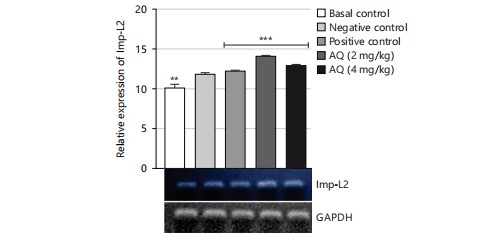
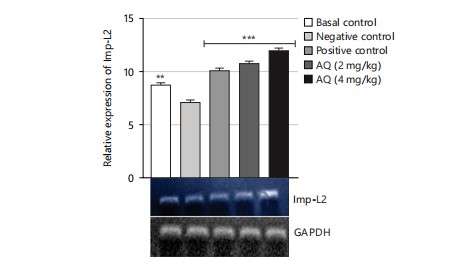
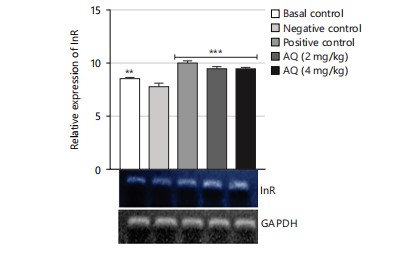
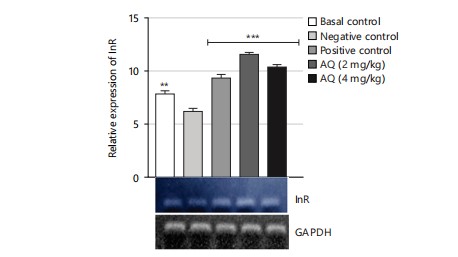
The gene expression analysis conducted in this study evaluated the effect of the AQ and EA fractions of Ficus exasperata leaf extract on the expression of the ILP-2, Imp-L2, and InR genes in Drosophila melanogaster. The ILP-2 is an important insulin-like peptide that plays a critical role in regulating energy homeostasis, glucose metabolism, and lifespan in insects38. Ecdysone (Imp-L2) plays a regulatory role by suppressing hunger in individuals with diabetes11. Diabetes and β-cell failure are the outcomes of peripheral insulin resistance brought on by systemic malfunction of insulin-like growth factor receptors (InR). On the other hand, the progression happens more quickly than in human type 2 diabetes mellitus39. Downregulating InR, however, is essential for improving glucose tolerance, avoiding diabetes, and maybe shielding β-cells from degeneration. Therefore, the upregulation of insulin-like growth factor substrate 2 could serve as a common mechanism for diabetes prevention or treatment40. Insulin receptor (InR) is a transmembrane receptor that plays a crucial role in insulin signaling in the body40,41. The receptor binds to insulin and initiates a cascade of signaling events that regulate glucose uptake and metabolism in various organs, such as the liver, muscle, and adipose tissue. The results of the gene expression analysis of the ethyl acetate fraction of Ficus exasperata on the InR gene showed that the plant extract upregulated the expression of the gene in a dose-dependent manner (Fig. 10-15).
|
|
The relative gene expression values in flies treated with extract fractions were higher than those of the negative control (without treatment), which also compares favorably with the positive control group (acarbose-treated flies). This suggests that the fractions of Ficus exasperata have the potential to enhance the expression of the insulin genes, which could be beneficial in improving glucose uptake and regulating insulin signaling in the body. In addition, the observed outcome, however, followed the trend of what was reported by Saliu et al.42 in which dietary intervention through the incorporation of plants is a major measure in combating the menace of diabetes. As a result, this present result will eventually present F. exasperata as part of many researched plants to be targeted for unraveling the mechanisms of managing diabetes.
|
|
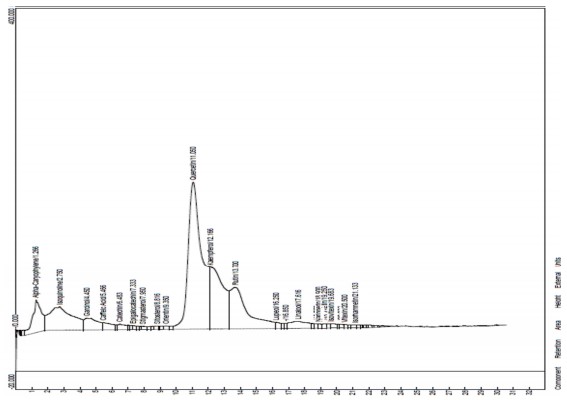
The HPLC analysis was done to determine the compounds that were present in the plant43. The HPLC results for the EA fraction reveal the presence of several significant compounds (Fig. 16 and Table 3). The biochemical functions of these bioactive natural components carry out a lot of therapeutic activities peculiar to them. Alpha-Caryophyllene (1.266%) is known for its potential anti-inflammatory and analgesic properties. Isoquinoline (2.750%) is an alkaloid that may have pharmacological effects. Garcinol (4.450%) is a polyisoprenylated benzophenone studied for its antioxidant and anticancer properties. Caffeic acid (5.466%) is a well-known phenolic acid with antioxidant properties. Catechin (6.483%) is a flavonoid with antioxidant properties, often found in tea. Epigallocatechin (7.333%) is another flavonoid commonly found in green tea, known for its antioxidant and potential anti-inflammatory effects43,44. Stigmasterol (7.950%) is a plant sterol that may have cholesterol-lowering properties. Sitosterol (8.816%) is another plant sterol often used as a dietary supplement with potential health benefits. Orientin (9.350%) is a flavonoid glycoside with antioxidant and anti-inflammatory properties. Quercetin (11.050%) is a potent flavonoid with antioxidant and anti-inflammatory effects, found in various fruits and vegetables. Kaempferol (12.166%) is a flavonoid with antioxidant and anti-inflammatory properties, commonly found in leafy greens, berries, and tea. Rutin (13.700%) is a flavonoid glycoside known for its antioxidant properties, often used as a dietary supplement.
|
|
Lupeol (16.250%) is a triterpene found in many plants, studied for potential anti-inflammatory and anticancer effects. Linalool (17.616%) is a terpene alcohol with a pleasant aroma, commonly found in the essential oils of various plants, known for its relaxing and anti-anxiety properties. Naringenin (18.900%) is a flavanone glycoside found in citrus fruits, with antioxidant and anti-inflammatory properties. Hesperidin (19.250%) is another flavanone glycoside commonly found in citrus fruits, known for its potential cardiovascular benefits. Isovitexin (19.683%) is a flavonoid with antioxidant and potential neuroprotective properties. Vitexin (20.500%) is a flavonoid with antioxidant and anti-inflammatory effects. Isorhamnetin (21.133%) is a flavonoid with antioxidant properties, often found in various fruits and vegetables45. These compounds exhibit diverse health benefits, including antioxidant, anti-inflammatory, and potential therapeutic properties. The percentages represent the relative abundance of each compound in Ficus exasperata leaves, providing valuable information for further research and understanding the plant’s potential health-promoting properties.
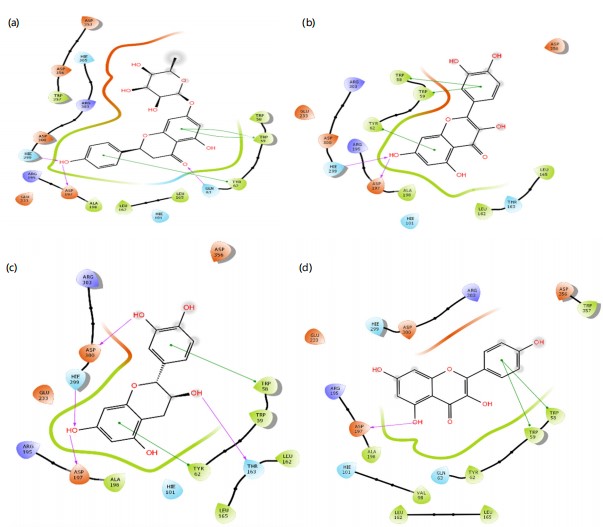
The prediction of potent antidiabetic molecules with high binding affinity against the binding pocket of α-amylase was carried out through the compounds identified from the HPLC chromatogram of plant extract, and were docked against the alpha-amylase using the Module of Schrodinger. The compounds showed varying degrees of binding affinities and MMGBSA scores for a-amylase. Naringenin (-7.118 and -41.270 kcal/mol), quercetin (-5.898 and -31.715 kcal/mol), catechin (-4.953 and -25.324l kcal/mol), and kaempferol (-4.814 and -27.561 kcal/mol), respectively, had higher docking scores against a-amylase among other identified compounds. In contrast, acarbose, a known antidiabetic drug, exhibited a strong docking score of -9.337 and an MMGBSA (Table 4). The ligands interacted with various amino acids present in the binding pockets of a-amylase with different molecular interactions such as van dal Waals, hydrogen bonds (Fig. 17). This was accomplished by analyzing the precise chemical interactions that the test drugs had with the protein targets’ binding pockets that allowed for inhibition. Naringenin interacts with the amino acid residues GLN 63, ASP 197, and HIE 299 through hydrogen bonds. Additionally, it interacts with amino acid residues TYR 62 and TRP 59 by pi-pi stacking. Quercetin forms hydrogen bond interactions with amino acid residues HIE 299 and ASP 197. It also it engages in pi-pi stacking interactions with amino acid residues TRP 58, TRP 59, and TRP 62. Catechin establishes hydrogen bond interactions with HIE 299, ASP 197, ASP 300, and THR 163. It also exhibits pi-pi stacking interactions with the amino acid residue TYR 62. Kaempferol forms hydrogen bond interactions with amino acid residue ASP 197 while it engages in pi-pi stacking interactions with amino acid residues TRP 58 and TRP 59. Acarbose is involved in multiple hydrogen bond interactions with amino acid residues, including HIE 305, ASP 356, ASP 300, HIE 299, THR 163, and ASP 197.
Molecular docking was done to evaluate the antidiabetic potential of Ficus exasperata by targeting alpha-amylase45,46. A binding energy calculation was also done to gain insight into their stability47. The results of molecular docking and binding energy calculations provide insights into the potential antidiabetic properties of compounds found in Ficus exasperata leaves (Table 3). Among the compounds tested, Naringenin exhibited the highest docking score of -7.118, suggesting a strong binding affinity to the target protein, alpha-amylase. The MMGBSA value of -41.270 further supports the idea that the binding of Naringenin to alpha-amylase is energetically favorable. These findings indicate that Naringenin may hold promise as an alpha-amylase inhibitor, potentially contributing to antidiabetic effects. Quercetin also showed a favorable docking score of -5.898 and an MMGBSA value of -31.715, indicating significant binding affinity to alpha-amylase. While not as potent as Naringenin, Quercetin still holds potential as an alpha-amylase inhibitor, suggesting possible antidiabetic properties. Catechin, with a docking score of -4.953 and an MMGBSA value of -25.324, demonstrates reasonable binding affinity to alpha-amylase. While its potency may not be as high as that of Naringenin or Quercetin, Catechin still suggests some potential as an alpha-amylase inhibitor, contributing to potential antidiabetic effects. Kaempferol, with a docking score of -4.814 and an MMGBSA value of -27.561, also shows reasonable binding affinity to alpha-amylase. These results suggest that Kaempferol may have the capacity to inhibit alpha-amylase and may contribute to the antidiabetic properties of Ficus exasperata. In contrast, Acarbose, a known antidiabetic drug, exhibited a strong docking score of -9.337 and an MMGBSA value of -42.270. These results validate the reliability of the docking and energy calculations in this study. This is also in line with the reported work of Akinnusi et al.48.
 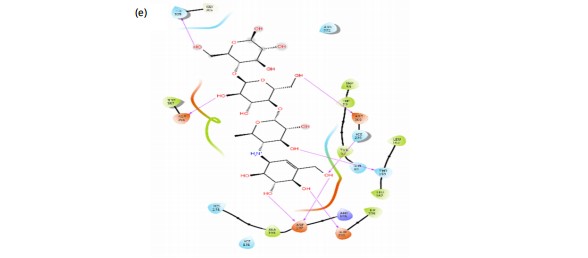
|
The ADMET study was done for the lead compounds to probe their physicochemical, pharmacokinetics, drug-likeness and toxicity properties48. Lipophilicity (Log P): Lipophilicity is a measure of a compound’s affinity for lipids or its hydrophobic character. In this case, all the compounds have Log P values close to zero, indicating a balanced hydrophilic and lipophilic character49. This is generally desirable for drug candidates because extreme values in either direction can pose challenges. Compounds that are too lipophilic may have issues with absorption, while those that are too hydrophilic may not distribute well in the body. Water Solubility (Log Sw): Water solubility is a critical factor in a compound’s bioavailability. All the compounds in the table are classified as “Soluble” by Silicos-IT, suggesting they have good water solubility. High water solubility is advantageous because it aids in the distribution of a compound in the body and its potential for therapeutic effects. Poor solubility can lead to lower bioavailability and, consequently, reduced effectiveness. These results indicate that all the compounds have a balanced character, with good solubility in water. This is a positive sign for their potential as lead compounds in drug development, as it suggests that they are more likely to be absorbed, distributed, and, therefore, bioavailable48. It’s important to note that lipophilicity and water solubility are just two factors in a comprehensive assessment of drug candidates. Other aspects, such as toxicity, pharmacological activity, and pharmacokinetics, would also need to be considered when evaluating these compounds for further development. In comparison to acarbose, these compounds seem to have more balanced physicochemical properties. acarbose has a notably lower Log P value, indicating higher hydrophilicity, and a much higher Log Sw value, suggesting very high water solubility. While these properties might be suitable for its intended use as an anti-diabetic medication, the other compounds show characteristics that are more balanced and potentially versatile for a broader range of applications in the pharmaceutical field49.
The Lipinski Rule of Five is a set of criteria used to evaluate the drug-likeness of a compound50. It focuses on parameters related to molecular weight, lipophilicity, and hydrogen bonding to identify compounds with a higher likelihood of successful oral absorption and bioavailability. The criteria involve no more than 5 hydrogen bond donors (expressed as the sum of OH and NH groups). No more than 10 hydrogen bond acceptors (expressed as N and O atoms). A molecular weight of less than 500 Daltons. A calculated Log P (lipophilicity) value is less than 5. Naringenin, Quercetin, Catechin, and Kaempferol: These four compounds have zero Lipinski rule violations. They meet all of Lipinski’s criteria, suggesting they have good drug-likeness based on these parameters. This is a positive indication of their potential as drug candidates, particularly for oral administration. Acarbose, on the other hand, has three Lipinski rule violations. It exceeds the acceptable limits for both hydrogen bond donors and acceptors, and it also has a relatively high molecular weight. These violations might suggest that acarbose could face challenges related to oral bioavailability according to Lipinski’s criteria.
The bioavailability score is a measure of a compound’s potential to be absorbed and become bioavailable when administered51. In this case, all four lead compounds have a bioavailability score of 0.55, while acarbose has a bioavailability score of 0.17. This signifies that 55% of each lead compound would be readily bioavailable in the cell and get to the site of action, while only 17% of acarbose would be readily bioavailable in the cell and get to the site of action. This result better validates the drug likeness result according to Lipinski, as discussed above. Based on the Lipinski Rule of Five, Naringenin, Quercetin, Catechin, and Kaempferol exhibit good drug-likeness. They meet all of Lipinski’s criteria, suggesting they have characteristics that are typically associated with successful oral absorption and bioavailability. Acarbose, however, violates three of the Lipinski rules, indicating potential challenges with its bioavailability according to these criteria.
The ADMET (Absorption, Distribution, Metabolism, Excretion, and Toxicity) property predictions for the same five compounds: Naringenin, Quercetin, Catechin, Kaempferol, and acarbose. These predictions are essential in evaluating the potential safety and efficacy of these compounds as drug candidates52. As for the prediction, naringenin is predicted to have low gastrointestinal (GI) absorption, which suggests that it may not be readily absorbed when taken orally. Quercetin, Catechin, and Kaempferol are predicted to have high GI absorption, indicating that they are more likely to be effectively absorbed from the gastrointestinal tract when taken orally. Acarbose is predicted to have low GI absorption, which could indicate potential issues with oral absorption. To the BBB Permeant (Blood-Brain Barrier Permeability), none of the compounds are predicted to be blood-brain barrier (BBB) permeants. This suggests that these compounds may have limited access to the central nervous system, which can be advantageous for some therapeutic applications by reducing the risk of central side effects52.
The Pgp is a transporter protein that can influence the absorption and distribution of drugs. Naringenin, quercetin, and acarbose are predicted to be P-glycoprotein (Pgp) substrates. Being a Pgp substrate may affect the bioavailability and distribution of these compounds. Catechin and Kaempferol are not predicted to be Pgp substrates, suggesting that they may not be as strongly influenced by Pgp-mediated efflux mechanisms.
About CYP Inhibition (Cytochrome P450 Inhibition), naringenin, quercetin, and acarbose are predicted to be CYP1A2 inhibitors, indicating that they may interfere with the activity of the cytochrome P450 enzyme CYP1A2, which plays a role in drug metabolism. Catechin and Kaempferol are not predicted to inhibit CYP1A2. Catechin and Kaempferol are predicted to inhibit CYP2C19, CYP2C9, and CYP2D6. This suggests that they may interfere with the metabolism of drugs metabolized by these enzymes. Naringenin and quercetin are not predicted to inhibit CYP2C19, CYP2C9, or CYP2D6. None of the compounds are predicted to inhibit CYP3A4, an enzyme involved in the metabolism of a wide range of drugs53. These predictions are crucial for understanding the compounds’ pharmacokinetics, metabolism, and potential drug-drug interactions, which are important considerations in drug development and safety.
The LD50 (mg/kg): The LD50 is a measure of acute toxicity and represents the dose at which 50% of a tested population is expected to die as a result of exposure to a substance54. A higher LD50 value indicates lower acute toxicity, while a lower LD50 value suggests higher toxicity. Naringenin: LD50 of 2300 mg/kg. This relatively high LD50 value suggests low acute toxicity for Naringenin. Quercetin’s LD50 of 10000 mg/kg. Quercetin also has a relatively high LD50 value, indicating low acute toxicity. Catechin’s LD50 of 159 mg/kg. Catechin has a significantly lower LD50, suggesting a higher level of acute toxicity compared to naringenin and quercetin. Kaempferol’s LD50 of 3919 mg/kg. Kaempferol falls in the moderate range of acute toxicity when compared to the other compounds. Acarbose’s LD50 of 24000 mg/kg. Acarbose, like quercetin, has a relatively high LD50, indicating low acute toxicity.
The compounds are assigned a toxicity class, with a higher number indicating higher predicted toxicity. It’s important to note that this classification is specific to the ProTox II model. Naringenin’s toxicity class 5 indicates a lower level of predicted toxicity. Quercetin’s toxicity class 6 suggests a lower level of predicted toxicity. Catechin’s toxicity is class 3, indicating a moderate level of predicted toxicity. Kaempferol’s toxicity class is 5, similar to Naringenin. Acarbose’s toxicity class is 6, like quercetin.
The summary of the predictions for each compound is shown below: Naringenin was not predicted to be hepatotoxic, carcinogenic, immunotoxin, mutagenic, or cytotoxic.
Quercetin was not predicted to have any of the listed toxicities. Catechin: Predicted to be immunotoxic and mutagenic, but not hepatotoxic, carcinogenic, or cytotoxic. Kaempferol was not predicted to have any of the listed toxicities. Acarbose was predicted to be carcinogenic, but not hepatotoxic, immunotoxic, mutagenic, or cytotoxic. The ProTox II toxicity predictions provide insights into the acute toxicity of these compounds. While naringerin, quercetin, kaempferol, and acarbose are predicted to have low acute toxicity, Catechin is predicted to have a higher level of acute toxicity. The predictions for specific types of toxicity (hepatotoxicity, carcinogenicity, immunotoxicity, mutagenicity, cytotoxicity) vary for each compound.
The comprehensive evaluation of the five compounds, naringenin, quercetin, catechin, kaempferol, and acarbose, reveals valuable insights into their potential as drug candidates in terms of their ADMET properties and toxicity predictions48. For ADMET properties, naringenin, quercetin, catechin, and kaempferol demonstrate favorable characteristics. They exhibit balanced lipophilicity (Log P values), high water solubility (Log Sw), and good drug-likeness based on Lipinski’s Rule of Five, and varying degrees of gastrointestinal absorption. Naringenin, Quercetin, and Kaempferol are also less likely to be substrates for P-glycoprotein (Pgp), which is a transporter known to influence drug distribution. In contrast, Acarbose shows lower drug-likeness based on Lipinski’s criteria and has some ADMET properties that may be less favorable.
Toxicity predictions: In terms of acute toxicity, Naringenin, Quercetin, Kaempferol, and Acarbose have relatively higher LD50 values, indicating lower acute toxicity. Catechin, however, has a lower LD50, suggesting a higher level of acute toxicity. The ProTox II predictions show that each compound has a unique profile of toxicity risks, with catechin and acarbose exhibiting potential concerns, particularly about to carcinogenicity and mutagenicity. Considering these findings, it can be inferred that naringenin, quercetin, and Kaempferol have more favorable overall ADMET profiles and lower predicted acute toxicity compared to acarbose. Catechin, while demonstrating good ADMET properties, raises concerns due to its higher predicted acute toxicity. Among the compounds evaluated, Quercetin stands out as a particularly strong candidate, as it combines favorable ADMET properties with low predicted acute toxicity.
CONCLUSION
This study demonstrates that both the aqueous and ethyl acetate fractions of Ficus exasperata leaf extract possess significant antioxidant and potential anti-diabetic properties. The ethyl acetate fraction, in particular, exhibited higher phenolic content, stronger antioxidant activity, and more pronounced upregulation of ILP-2, InR, and Imp-L2 gene expression in Drosophila flies, comparable to acarbose treatment. In silico analysis identified naringenin, quercetin, catechin, and kaempferol as lead compounds, with naringenin and quercetin showing superior ADMET profiles over the standard drug. These findings highlight the therapeutic promise of F. exasperata fractions in managing type 2 diabetes and related metabolic disorders. Nonetheless, further in-depth studies are essential to establish their safety, efficacy, and full pharmacological potential.
SIGNIFICANCE STATEMENT
This study identified naringenin and quercetin as bioactive compounds from Ficus exasperata leaf extract fractions, which could be beneficial for the management of diabetes mellitus and its related oxidative stress complications. This study will assist researchers in uncovering critical areas of plant-based insulin signaling modulation and natural antioxidant therapy that have remained unexplored by many. Consequently, a new theory on the integration of phytochemicals in diabetes management and metabolic disorder therapeutics may be developed.
REFERENCES
- Mobasseri, M., M. Shirmohammadi, T. Amiri, N. Vahed, H.H. Fard and M. Ghojazadeh, 2020. Prevalence and incidence of type 1 diabetes in the world: A systematic review and meta-analysis. Health Promot. Perspect., 10: 98-115.
- Vos, T., S.S. Lim, C. Abbafati, K.M. Abbas and M. Abbasi et al., 2020. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet, 396: 1204-1222.
- Onyango, E.M. and B.M. Onyango, 2018. The rise of noncommunicable diseases in Kenya: An examination of the time trends and contribution of the changes in diet and physical inactivity. J. Epidemiol. Global Health, 8: 1-7.
- Uloko, A.E., B.M. Musa, M.A. Ramalan, I.D. Gezawa and F.H. Puepet et al., 2018. Prevalence and risk factors for diabetes mellitus in Nigeria: A systematic review and meta-analysis. Diabetes Ther., 9: 1307-1316.
- Yesufu, J.O., O.D. Oluwasile, O.I. Oluranti, A.A. Fasanmade and A.O. Soladoye, 2020. Cardiopulmonary health indices and diabetes risk scores in undergraduate students of a private university in Nigeria. Beni-Suef Univ. J. Basic Appl. Sci., 9.
- Ojo, O.A., J.C. Amanze, A.I. Oni, S. Grant and M. Iyobhebhe et al., 2022. Antidiabetic activity of avocado seeds (Persea americana Mill.) in diabetic rats via activation of PI3K/AKT signaling pathway. Sci. Rep., 12.
- Bai, Y., K. Li, J. Shao, Q. Luo and L.H. Jin, 2018. Flos Chrysanthemi Indici extract improves a high-sucrose diet‑induced metabolic disorder in Drosophila. Exp. Ther. Med., 16: 2564-2572.
- Graham, P. and L. Pick, 2017. Drosophila as a Model for Diabetes and Diseases of Insulin Resistance. In: Current Topics in Developmental Biology, Pick, L. (Ed.), Academic Press, Cambridge, Massachusett, ISBN: 9780128029046, pp: 397-419.
- Goldfine, I.D. and J.F. Youngren, 1998. Contributions of the American Journal of Physiology to the discovery of insulin. Am. J. Physiol. Endocrinol. Metab., 274: E207-E209.
- Álvarez-Rendón, J.P., R. Salceda and J.R. Riesgo-Escovar, 2018. Drosophila melanogaster as a model for diabetes type 2 progression. BioMed Res. Int., 2018.
- Honegger, B., M. Galic, K. Köhler, F. Wittwer, W. Brogiolo, E. Hafen and H. Stocker, 2008. Imp-L2, a putative homolog of vertebrate IGF-binding protein 7, counteracts insulin signaling in Drosophila and is essential for starvation resistance. J. Biol., 7.
- Birse, R.T., J. Choi, K. Reardon, J. Rodriguez and S. Graham et al., 2010. High-fat-diet-induced obesity and heart dysfunction are regulated by the TOR pathway in Drosophila. Cell Metab., 12: 533-544.
- Musselman, L.P., J.L. Fink, K. Narzinski, P.V. Ramachandran, S.S. Hathiramani, R.L. Cagan and T.J. Baranski, 2011. A high-sugar diet produces obesity and insulin resistance in wild-type Drosophila. Dis. Model. Mech., 4 : 842-849.
- Kooti, W., M. Farokhipour, Z. Asadzadeh, D. Ashtary-Larky and M. Asadi-Samani, 2016. The role of medicinal plants in the treatment of diabetes: A systematic review. Electron. Phys., 8: 1832-1842.
- Prabhakar, P.K. and M. Doble, 2008. A target based therapeutic approach towards diabetes mellitus using medicinal plants. Curr. Diabetes Rev., 4: 291-308.
- Triantafillidis, J., A. Triantafyllidi, C. Vagianos and A. Papalois, 2016. Favorable results from the use of herbal and plant products in inflammatory bowel disease: Evidence from experimental animal studies. Ann. Gastroenterol., 29: 268-281.
- Adekeye, A.O., G.J. Irawo and A.A. Fafure, 2020. Ficus exasperata Vahl leaves extract attenuates motor deficit in vanadium-induced parkinsonism mice. Anat. Cell Biol., 53: 183-193.
- Ogunleye, D.S., A.A. Adeyemi and A.M. Sanni, 2003. Hypoglycaemic activities of the stem bark of Cola Acuminata VAHL and leaf of Ficus exasperata (P.Beauv) SCHOTT and ENDL. Niger. Q. J. Hosp. Med., 13: 58-60.
- Ayinde, B.A., E.K. Omogbai and F.C. Amaechina, 2007. Pharmacognosy and hypotensive evaluation of Ficus exasperata Vahl (Moraceae) leaf. Acta Poloniae Pharm., 64: 543-546.
- Singleton, V.L., R. Orthofer and R.M. Lamuela-Raventós, 1999. Analysis of Total Phenols and other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In: Methods in Enzymology, Packer, L. (Ed.), Academic Press, Cambridge, Massachusetts, ISBN: 9780121822002, pp: 152-178.
- Meda, A., C.E. Lamien, M. Romito, J. Millogo and O.G. Nacoulma, 2005. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem., 91: 571-577.
- Ghorai, N., S. Chakraborty, S. Gucchait, S.K. Saha and S. Biswas, 2012. Estimation of total terpenoids concentration in plant tissues using a monoterpene, linalool as standard reagent. Protoc. Exch.
- Oyaizu, M., 1986. Studies on products of browning reaction: Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. Diet., 44: 307-315.
- Gyamfi, M.A., M. Yonamine and Y. Aniya, 1999. Free-radical scavenging action of medicinal herbs from Ghana: Thonningia sanguinea on experimentally-induced liver injuries. Gen. Pharmacol.: Vasc. Syst., 32: 661-667.
- Omale, S., J.C. Aguiyi, O.G. Adekunle, T.O. Johnson, S.O. Ochala, M.A. Etuh and M.C. Eze, 2021. Evaluation of the antidiabetic effects of the stem bark extract of Parinari curatellifolia (Planch. ex Benth.) in Drosophila melanogaster. J. Pharmacol. Toxicol., 16: 9-21.
- Adedara, A.O., A.D. Babalola, F. Stephano, I.O. Awogbindin and J.O. Olopade et al., 2022. An assessment of the rescue action of resveratrol in parkin loss of function-induced oxidative stress in Drosophila melanogaster. Sci. Rep., 12.
- Trinder, P., 1969. Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann. Clin. Biochem., 6: 24-27.
- Abolaji, A.O., C.O. Olaiya, O.J. Oluwadahunsi and E.O. Farombi, 2017. Dietary consumption of monosodium L-glutamate induces adaptive response and reduction in the life span of Drosophila melanogaster. Cell Biochem. Funct., 35: 164-170.
- Green, L.C., D.A. Wagner, J. Glogowski, P.L. Skipper, J.S. Wishnok and S.R. Tannenbaum, 1982. Analysis of nitrate, nitrite and [15N]nitrate in biological fluids. Anal. Biochem., 126: 131-138.
- Patterson, B.D., E.A. MacRae and I.B. Ferguson, 1984. Estimation of hydrogen peroxide in plant extracts using titanium(IV). Anal. Biochem., 139: 487-492.
- Balogun, T.A., N. Ipinloju, O.T. Abdullateef, S.I. Moses, D.A. Omoboyowa et al., 2021. Computational evaluation of bioactive compounds from Colocasia affinis Schott as a novel EGFR inhibitor for cancer treatment. Cancer Inf., 20.
- Noreen, H., N. Semmar, M. Farman and J.S.O. McCullagh, 2017. Measurement of total phenolic content and antioxidant activity of aerial parts of medicinal plant Coronopus didymus. Asian Pac. J. Trop. Med., 10: 792-801.
- Kaurinovic, B. and D. Vastag, 2019. Flavonoids and Phenolic Acids as Potential Natural Antioxidants. In: Antioxidants, Shalaby, E. and T. Brzozowski (Eds.), IntechOpen, London, United Kingdom, ISBN: 978-1-78923-920-1.
- Krentz, A.J. and C.J. Bailey, 2005. Oral antidiabetic agents: Current role in type 2 diabetes mellitus. Drugs, 65: 385-411.
- Salbitani, G., V. Maresca, P. Cianciullo, R. Bossa, S. Carfagna and A. Basile, 2023. Non-protein thiol compounds and antioxidant responses involved in bryophyte heavy-metal tolerance. Int. J. Mol. Sci., 24.
- Prakash, M., M.S. Shetty, P. Tilak and N. Anwar, 2009. Total thiols: Biomedical importance and their alteration in various disorder. Online J. Health Allied Sci., 8.
- Bhatti, J.S., A. Sehrawat, J. Mishra, I.S. Sidhu and U. Navik et al., 2022. Oxidative stress in the pathophysiology of type 2 diabetes and related complications: Current therapeutics strategies and future perspectives. Free Radical Biol. Med., 184: 114-134.
- Chowański, S., K. Walkowiak-Nowicka, M. Winkiel, P. Marciniak, A. Urbański and J. Pacholska-Bogalska, 2021. Insulin-like peptides and cross-talk with other factors in the regulation of insect metabolism. Front. Physiol., 12.
- White, M.F., 2002. IRS proteins and the common path to diabetes. Am. J. Physiol. Endocrinol. Metab., 283: E413-E422.
- Hennige, A.M., D.J. Burks, U. Ozcan, R.N. Kulkarni and J. Ye et al., 2003. Upregulation of insulin receptor substrate-2 in pancreatic β cells prevents diabetes. J. Clin. Invest., 112: 1521-1532.
- Chen, J., Y. Huang and G. Qi, 2022.
LncRNA-IRAR -mediated regulation of insulin receptor transcripts in Drosophila melanogaster during nutritional stress. Insect Mol. Biol., 31: 261-272. - Saliu, J.A., A.M. Olajuyin and A. Akinnubi, 2021. Modulatory effect of Artocarpus camansi on ILP-2, InR, and Imp-L2 genes of sucrose-induced diabetes mellitus in Drosophila melanogaster. Comp. Biochem. Physiol. C: Toxicol. Pharmacol., 246.
- Shodehinde, S., I. Dasappa, P. Pichan, S. Olubode and P. Akinnusi, 2022. Comparison of nutritional composition, HPLC characterization, antioxidants property and starch profile of Sphenostylis stenocarpa composite bread and wheat bread. J. Med. Herbs, 3: 39-48.
- Hasanein, P., A. Emamjomeh, N. Chenarani and M. Bohlooli, 2020. Beneficial effects of rutin in diabetes-induced deficits in acquisition learning, retention memory and pain perception in rats. Nutr. Neurosci., 23: 563-574.
- Dewanjee, S., A.K. Das, R. Sahu and M. Gangopadhyay, 2009. Antidiabetic activity of Diospyros peregrina fruit: Effect on hyperglycemia, hyperlipidemia and augmented oxidative stress in experimental type 2 diabetes. Food Chem. Toxicol., 47: 2679-2685.
- Akinnusi, P.A., S.O. Olubode, A.A. Alade, A.A. Ashimi and O.L. Onawola et al., 2023. Potential inhibitory biomolecular interactions of natural compounds with different molecular targets of diabetes. Bioinf. Biol. Insights, 17.
- Akinnusi, P.A., S.O. Olubode, A.O. Adebesin, T.A. Nana and S.A. Shodehinde, 2022. Discovery of promising inhibitors of epidermal growth factor receptor (EGFR), human epidermal growth factor receptor 2 (HER2), estrogen receptor (ER), and phosphatidylinositol-3-kinase a (PI3Ka) for personalized breast cancer treatment. Cancer Inf., 21.
- Akinnusi, P.A., S.O. Olubode and W.A. Salaudeen, 2022. Molecular binding studies of anthocyanins with multiple antiviral activities against SARS-CoV-2. Bull. Natl. Res. Cent., 46.
- Akinnusi, P.A., S.O. Olubode, A.A. Alade, S.A. Ahmed and S.F. Ayekolu et al., 2022. A molecular modeling approach for structure-based virtual screening and identification of novel anti-hypercholesterolemic agents from grape. Inf. Med. Unlocked, 32.
- Lipinski, C.A., 2004. Lead- and drug-like compounds: The rule-of-five revolution. Drug Discovery Today: Technol., 1: 337-341.
- Olubode, S.O., M.O. Bankole, P.A. Akinnusi, O.S. Adanlawo and K.I. Ojubola et al., 2022. Molecular modeling studies of natural inhibitors of androgen signaling in prostate cancer. Cancer Inf., 21.
- Olubode, S., O. Bankole, P. Akinnusi, W. Salaudeen and K. Ojubola et al., 2022. Computational study of the inhibitory potential of Gongronema latifolium (Benth) leave on farnesyl pyrophosphate synthase, a target enzyme in the treatment of osteoporosis. A molecular modelling approach. J. Med. Herbs, 13: 39-47.
- Salehi, B., P.V.T. Fokou, M. Sharifi-Rad, P. Zucca, R. Pezzani, N. Martins and J. Sharifi-Rad, 2019. The therapeutic potential of naringenin: A review of clinical trials. Pharmaceuticals, 12.
- Adebesin, A.O., A.O. Ayodele, O. Omotoso, P.A. Akinnusi and S.O. Olubode, 2022. Computational evaluation of bioactive compounds from Vitis vinifera as a novel β-catenin inhibitor for cancer treatment. Bull. Natl. Res. Cent., 46.
How to Cite this paper?
APA-7 Style
Shodehinde,
S.A., Awojulu,
V.O., Nwanna,
E.E., Olubode,
S.O., Bello,
L., Akinnusi,
P.A., Adefolalu,
A., Awelewa,
O.V., Olubode,
S.O., Bakare,
O.S., Adetokunbo,
T., Egunjobi,
G.D. (2025). Multimodal Assessment of Ficus exasperata in Diabetic Drosophila. International Journal of Biological Chemistry, 19(1), 36-61. https://doi.org/10.3923/ijbc.2025.36.61
ACS Style
Shodehinde,
S.A.; Awojulu,
V.O.; Nwanna,
E.E.; Olubode,
S.O.; Bello,
L.; Akinnusi,
P.A.; Adefolalu,
A.; Awelewa,
O.V.; Olubode,
S.O.; Bakare,
O.S.; Adetokunbo,
T.; Egunjobi,
G.D. Multimodal Assessment of Ficus exasperata in Diabetic Drosophila. Int. J. Biol. Chem 2025, 19, 36-61. https://doi.org/10.3923/ijbc.2025.36.61
AMA Style
Shodehinde
SA, Awojulu
VO, Nwanna
EE, Olubode
SO, Bello
L, Akinnusi
PA, Adefolalu
A, Awelewa
OV, Olubode
SO, Bakare
OS, Adetokunbo
T, Egunjobi
GD. Multimodal Assessment of Ficus exasperata in Diabetic Drosophila. International Journal of Biological Chemistry. 2025; 19(1): 36-61. https://doi.org/10.3923/ijbc.2025.36.61
Chicago/Turabian Style
Shodehinde, Sidiqat, Adamson, Victor Oluwadamlola Awojulu, Esther Emem Nwanna, Samuel Olawale Olubode, Lateef Bello, Precious Ayorinde Akinnusi, Aderemi Adefolalu, Olamide Vincent Awelewa, Success Oluwaferanmi Olubode, Oluwafemi Shittu Bakare, Tobiloba Adetokunbo, and Gladys Diekonifelouwa Egunjobi.
2025. "Multimodal Assessment of Ficus exasperata in Diabetic Drosophila" International Journal of Biological Chemistry 19, no. 1: 36-61. https://doi.org/10.3923/ijbc.2025.36.61

This work is licensed under a Creative Commons Attribution 4.0 International License.



